Most radiotracers for PET imaging are utilized to assess their end-product properties, for instance, using F18-FDG to assess glucose metabolism. The intermediate molecular transport processes of radiotracers across a vascular barrier (e.g., blood-brain barrier) are much less explored. However, the transport steps of radiotracers may provide rich information on health and disease. A major focus of our lab is to develop PET methods to measure the molecular transport properties of a radiotracer, which may then be combined with their regular use to enable single-tracer multiparametric imaging. 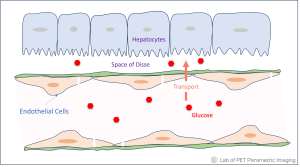 The enabling techniques include (a) dynamic PET imaging using high-performance scanners (e.g., EXPLORER total-body PET) or advanced image reconstruction methods (e.g., the kernel methods Li & Wang 2022, Wang 2019, Wang & Qi 2015), and (b) tracer kinetic modeling that allows more accurate quantification of the transport rates of a radiotracer in different organs.
The enabling techniques include (a) dynamic PET imaging using high-performance scanners (e.g., EXPLORER total-body PET) or advanced image reconstruction methods (e.g., the kernel methods Li & Wang 2022, Wang 2019, Wang & Qi 2015), and (b) tracer kinetic modeling that allows more accurate quantification of the transport rates of a radiotracer in different organs.
Delivery Rate K1. Since 2016, we have devoted our efforts to quantifying the blood-to-tissue delivery rate (K1) of radiotracers and exploring novel clinical applications. We tailored technical methods for K1 quantification in various organs, including the liver (Wang et al 2018, Zuo et al. 2019 ), heart (Zuo et al 2020), bone marrow (Wang Y et al. 2023), lungs (Wang Y et al 2024), and brain (Zhu et al 2024). Using the widely available radiotracer F18-FDG and closely collaborating with clinicians, we have explored the K1 imaging method for novel clinical applications:
- Assess liver inflammation in metabolic dysfunction-associated steatohepatitis (MASH) (Sarkar et al 2019, Sarkar et al 2021);
- Measure myocardial blood flow in patients with heart disease (Zuo et al 2021).
Quantitative Blood Flow by High-temporal Resolution Imaging. Despite their clinical potential, one limitation of the current K1 methods is that the estimates often represent a combination of blood flow and tracer-specific transport rates. It would be difficult to understand the underlying biology – how much is due to blood flow and how much is due to the molecularly specific transport step. To address this problem, we have developed methods that utilize high-temporal resolution dynamic PET imaging and advanced kinetic modeling to separate blood flow from tracer-specific transport rate in modeling the tracer delivery process (Chung et al 2024a; Wang et al. 2019; Wang et al. 2018). This method enables the technical ability to measure quantitative blood flow directly from the same scan for many, if not all, existing radiotracers (Chung et al 2024a).
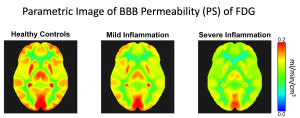 Radiotracer Transport and Permeability. The high-temporal resolution method has also enabled the ability to quantify the tracer-specific molecular transport rate across vascular barriers more accurately (Wang et al. 2019). Further, this method also facilitates the measurement of the molecular permeability of radiotracers (Chung et al 2024b). The significance is illustrated through an example of assessing the blood-brain barrier (BBB) in patients with chronic liver inflammation. While cerebral blood flow remains statistically similar, the BBB transport rate and permeability surface area product (PS) of F18-FDG may differ significantly across liver inflammation grades (see the figure; Chung et al 2024b). This method opens new directions to efficiently study the molecular transport and permeability of the human BBB in vivo using a wide range of available molecular PET tracers.
Radiotracer Transport and Permeability. The high-temporal resolution method has also enabled the ability to quantify the tracer-specific molecular transport rate across vascular barriers more accurately (Wang et al. 2019). Further, this method also facilitates the measurement of the molecular permeability of radiotracers (Chung et al 2024b). The significance is illustrated through an example of assessing the blood-brain barrier (BBB) in patients with chronic liver inflammation. While cerebral blood flow remains statistically similar, the BBB transport rate and permeability surface area product (PS) of F18-FDG may differ significantly across liver inflammation grades (see the figure; Chung et al 2024b). This method opens new directions to efficiently study the molecular transport and permeability of the human BBB in vivo using a wide range of available molecular PET tracers.
Selected Publications:
- , , , , , , , , , , , ,
- Chung KJ, Chaudhari AJ, Nardo L, Jones T, Chen MS, Badawi RD, Cherry SR, Wang GB.
Quantitative total-body imaging of blood flow with high temporal resolution early dynamic 18F-Fluorodeoxyglucose PET kinetic modeling.
Journal of Nuclear Medicine, accepted, April 2025.
[Preprint: medRxiv doi: https://doi.org/10.1101/2024.08.30.24312867] - Wang Y, Nardo L, Spencer BA, Abdelhafez Y, Li EJ, Omidvari N, Chaudhari AJ, Badawi RD, Jones T, Cherry SR, and Wang GB.
Total-Body Multiparametric PET Quantification of 18F-FDG Delivery and Metabolism in the Study of COVID-19 Recovery.
Journal of Nuclear Medicine, , 2023. - Sarkar S, Matsukuma KE, Spencer B, Chen S, Olson KA, Badawi RD, Corwin MT, Wang GB.
Dynamic positron emission tomography/computed tomography imaging correlate of non-alcoholic steatohepatitis.
Clinical Gastroenterology and Hepatology, 19(11): 2441-2443, 2021. - Zuo Y, Lopez JE, Smith T, Foster CC, Carson RE, Badawi RD, Wang GB.
Multiparametric Cardiac 18F-FDG PET in Humans: Pilot Comparison of FDG Delivery Rate with 82Rb Myocardial Blood Flow.
Physics in Medicine and Biology, 66(15): 155015, July 2021. - Zuo Y, Badawi RD, Foster CC, Smith T, López JE, Wang GB.
Multiparametric cardiac 18F-FDG PET in humans: kinetic model selection and identifiability analysis.
IEEE Transactions on Radiation in Plasma and Medical Sciences, 4(6): 759 – 767, 2020. - Sarkar S, Corwin MT, Olson KA, Stewart S, Liu CH, Badawi RD, and Wang GB.
Pilot study to diagnose non-alcoholic steatohepatitis with dynamic 18F-fluorodeoxyglucose positron emission tomography.
American Journal of Roentgenology, 212(3): 529-537, 2019. - , , , , Dynamic PET of human liver inflammation: impact of kinetic modeling with optimization-derived dual-blood input function.
Physics in Medicine and Biology, 63(15): 155004 (14pp), 2018.
Funding Support:
This research is supported by the NIH R01 EB033435 from NIBIB and R01 DK124803 from NIDDK.