Clinical significance. This project aims to develop a liver parametric PET methodology to fill the gap in clinical imaging of fatty liver disease. Hepatic steatosis (fat), inflammation, and fibrosis are three major types of pathology in the wide spectrum of nonalcoholic fatty liver disease (NAFLD), affecting approximately 25% of the general population worldwide. Noninvasive imaging methods have been developed to quantify hepatic steatosis, e.g., by ultrasonography, CT or MRI, and to quantify liver fibrosis e.g., by MR elastography and ultrasound elastography. However, imaging methods remain limited for accurate characterization of liver inflammation. Invasive liver biopsy is the current standard practice.
We and clinical collaborators (Dr. Souvik Sarkar et al.) have for the first time demonstrated that dynamic 18F-FDG PET with new liver kinetic modeling can effectively assess hepatic inflammation in patients with NAFLD, thus opening the opportunity of developing a new PET-based noninvasive imaging method to fulfill the unmet need for differentiating nonalcoholic steatohepatitis (NASH, a more severe form of NAFLD) from simple steatosis.
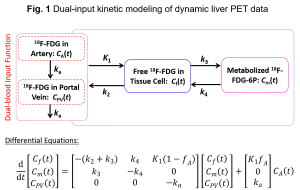
Optimization-derived dual-input kinetic modeling. The liver is unique because it has two blood supplies – the hepatic artery and portal vein, while most other organs are only supplied by the artery. Modeling the dual-blood input function (DBIF) is essential in liver tracer kinetic modeling for accurate quantification. A common approach is to use a flow-weighted model of DBIF with the model parameters often pre-determined by population means that were derived using arterial blood sampling in animal studies. Due to heterogeneity across patients and potential differences between arterial and image-derived input functions, the population-based DBIF model may become ineffective when arterial blood sampling is not available and in practice, an image-derived input function is typically used in human studies. We have developed a new optimization-derived DBIF model (Fig. 1) that employs mathematical optimization to jointly estimate the parameters of DBIF and liver kinetics (Wang et al., Phys. Med. Biol 2018). This model directly utilizes image-derived aortic input function, requires no arterial blood sampling, and is more adaptive to individual patients. We continue to investigate the theoretical and practical identifiability of the model (Zuo et al., Phys. Med. Biol. 2019) and develop new optimization forms to improve the modeling approach.
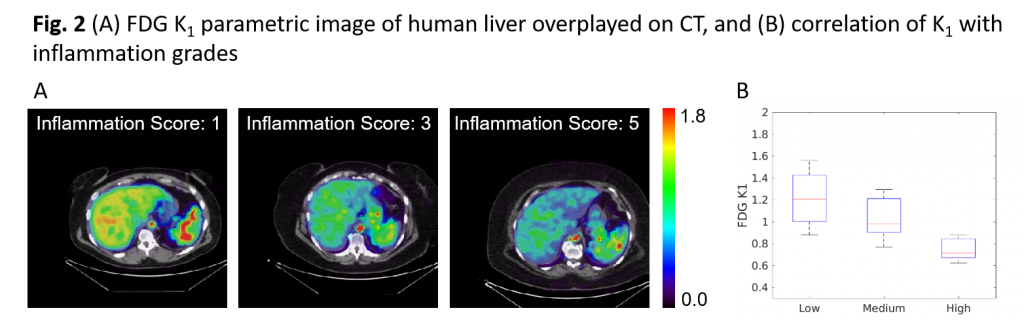
Discovering and understanding the glucose transport hypothesis for Liver Inflammation. With improved kinetic modeling, we have discovered that the rate of glucose transport from blood to hepatic tissue (denoted as 18F-FDG K1) correlates with the grades of liver inflammation with statistical significance (Fig. 2) (Sarkar et al. CGH 2021, Sarkar et al. AJR, 2019, Wang et al., Phys. Med. Biol 2018). 18F-FDG K1 achieved the highest differentiation power for detecting NASH among different imaging biomarkers. While K1 may consist of multiple physiological processes, we are conducting studies to understand further the potential glucose transport hypothesis for liver inflammation in NAFLD.
Multiparametric imaging of liver inflammation, fat, and fibrosis. With the ability to quantify liver inflammation using dynamic 18F-FDG PET, we are currently also developing and evaluating the integrated assessment of both liver fat (with CT) and inflammation (with PET) using PET/CT (Duan et al, JNM 2025, Sarkar et al. CGH 2021). The same principle can also be extended to PET/MR. This development will allow a “one-stop-shop” tool for NASH diagnosis.
Funding Support:
This project is supported by NIH under no. R01DK124803.
Collaborators at UCD Medical Center:
Hepatology: Souvik Sarkar (MD, PhD), Valentina Medici (MD)
Radiology: Michael T. Corwin (MD), Ramsey D. Badawi (PhD)
Pathology: Karen E. Matsukuma (MD), Kristin A. Olson (MD)
Bariatric Surgery: Victoria Lyo (MD)
Selected Publications:
- Duan X, Sarkar S, Lyo V, Romeo S, Spencer BA, Matsukuma KE, Medici V, Corwin MT, Badawi RD, and Wang GB.
Quantification of 18F-FDG delivery rate for liver inflammation using shortened dynamic PET imaging protocols.
Journal of Nuclear Medicine, accepted, August 2025. - Sarkar S, Matsukuma KE, Spencer B, Chen S, Olson KA, Badawi RD, Corwin MT, Wang GB.
Dynamic PET/CT imaging correlate of non-alcoholic steatohepatitis.
Clinical Gastroenterology and Hepatology, 19(11): 2441-2443, 2021. - Zuo Y, Sarkar S, Corwin MT, Olson K, Badawi RD, Wang GB.
Structural and practical identifiability of dual-input kinetic modeling in dynamic PET of liver inflammation.
- Sarkar S, Corwin MT, Olson KA, Stewart S, Liu CH, Badawi RD, and Wang GB.
Pilot study to diagnose non-alcoholic steatohepatitis with dynamic 18F-fluorodeoxyglucose positron emission tomography.
American Journal of Roentgenology, 212(3): 529-537, 2019. - , , , ,
News Report:
2021: UC Davis Health Newsroom; Top Story on SNMMI SmartBrief