Clinical Significance. Myocardial perfusion imaging with PET has been routinely used in the clinic with a flow-specific radiotracer (e.g., 82Rb-chloride or 13N-ammonia). However, it suffers from limited clinical availability due to the short half-life of the radiotracers. On the other hand, 18F-fluorodeoxyglucose (FDG) is the most accessible radiotracer for PET and has been used in clinical cardiology mainly for assessing glucose metabolism. The combined use of FDG (for metabolic imaging) and 82Rb-chloride (or 13N-ammonia, for myocardial perfusion imaging) is the clinical gold standard for determining myocardial viability in patients with ischemic cardiomyopathy (a form of heart failure), but this dual-tracer method is resource-intensive and also cost-ineffective.
In this project, we explore the capability of the classic metabolic radiotracer 18F-FDG for assessing myocardial blood flow. We develop methods of dynamic imaging and tracer kinetic modeling to measure quantitative blood flow from dynamic FDG-PET data. This “myocardial FDG flow” method can be used alone for myocardial perfusion imaging or is combined with FDG metabolism imaging to enable single-tracer (i.e. FDG) multiparametric (i.e. flow-metabolism) imaging in the myocardium without the need for a flow-specific radiotracer.
Challenges. Our early attempt used myocardial FDG K1 – the delivery rate of FDG transport from blood to myocytes – as a surrogate of myocardial blood flow. We investigated the appropriate dynamic scan and kinetic modeling protocols for efficient quantification of myocardial FDG K1 (Zuo et al IEEE Trans. RPMS 2020). However, FDG is not a freely diffusible radiotracer but instead relies on facilitated transport via glucose transporters (GLUTs). FDG K1 represents a mix of blood flow and glucose transport. It can approximate blood flow accurately for regions of high FDG extraction fraction (e.g., high-grade tumors), but is ineffective in regions of low or medium FDG extraction fraction, such as in most organ tissues including the myocardium. In addition, myocardial FDG K1 can be influenced by blood glucose levels, as demonstrated in our recent study (Zuo et al Phys. Med. Biol. 2021).
Methodological Developments. We have tackled these problems by studying and correcting the effects of blood glucose levels and FDG extraction fraction in the myocardium (Zuo et al Phys. Med. Biol. 2021).
- Glucose Normalization. Our result from a pilot human patient study demonstrated that myocardial FDG K1 correlates with blood sugar levels inversely. We then proposed glucose normalization algorithms to calibrate myocardial FDG K1 to a reference blood glucose level. In this way, the dependence of myocardial FDG K1 on blood glucose is diminished. The glucose-normalized myocardial FDG K1 becomes more closely associated with myocardial blood flow as assessed by dynamic 82Rb-PET.
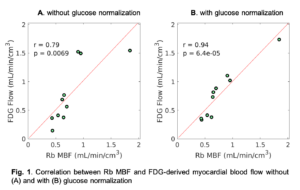
- FDG Extraction Fraction Correction. Our study also showed that the extraction fraction of FDG in the myocardium is close to that of 82Rb-chloride. Using the generalized Renkin-Crone model, we applied a nonlinear extraction fraction correction to convert glucose-normalized myocardial FDG K1 to quantitative myocardial blood flow. Fig. 1 shows a correlation of FDG-derived myocardial blood flow (with or without glucose normalization) with 82Rb myocardial blood flow at resting state.
Our ongoing effort is to further develop and validate the method for cardiac rest-stress imaging.
This project was supported in part by NIH R21 HL131385 and AHA BGIA25780046.
Journal Papers:
- Zuo Y, Lopez JE, Smith T, Foster CC, Carson RE, Badawi RD, Wang GB.
Multiparametric cardiac 18F-FDG PET in humans: Pilot comparison of FDG delivery rate with 82Rb myocardial blood flow.
Physics in Medicine and Biology, 66(15): 155015, July 2021.
[Preprint: arXiv:2010.12724] - Zuo Y, Badawi RD, Foster CC, Smith T, López JE, Wang GB.
Multiparametric cardiac 18F-FDG PET in humans: kinetic model selection and identifiability analysis.
IEEE Transactions on Radiation in Plasma and Medical Sciences (TRPMS), 2020, 4(6): 759 – 767.
[Preprint: arXiv:2008.05099. August 2020]