Current clinical PET can provide an effective temporal resolution of 5-10 seconds but with compromised image quality. This temporal resolution can be adequate for traditional compartmental modeling that assumes uniform concentrations in compartments but becomes inadequate for exploiting the benefit of more advanced kinetic models that account for the heterogeneous behaviors of radiotracer transport. We aim to explore higher temporal resolution imaging, for example 1-2 seconds with current clinical PET scanners and sub-second with high-sensitivity PET scanners (e.g., the EXPLORER total-body PET).
We develop advanced image reconstruction to improve HTR dynamic PET imaging and develop new kinetic modeling strategies to exploit HTR data for radiotracer transport imaging. 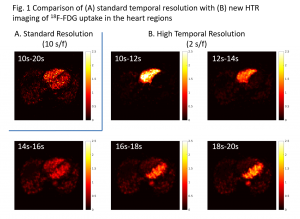
HTR Image Reconstruction: A major challenge associated with HTR dynamic PET imaging is the reduced counting statistics (high noise) in each short-time frame. We are developing novel image reconstruction algorithms to overcome the noise challenge. One example of our recent work is the spatiotemporal kernel method for HTR image reconstruction (Wang, IEEE-TMI 2019, bioRxiv 2018; Fully3D 2017), which continues our previous development on the spatial kernel method for standard dynamic PET imaging (Wang&Qi, IEEE-TMI 2015). These kernel methods for tomographic image reconstruction are inspired by kernel machines in machine learning. Figures 1 and 2 show a comparison between standard 10-s temporal resolution (by standard reconstruction method) and new 2-s HTR imaging by the spatiotemporal kernel method for a real patient 18F-FDG PET scan. Despite the relatively old scanner (GE Discovery ST), rapid activity changes in the ventricles can be captured by the kernel-based HTR imaging without suffering from noise.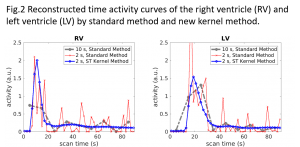
HTR Kinetic Modeling: We are developing new kinetic modeling strategies to better utilize HTR data for radiotracer transport imaging. One example is the work of time-varying kinetic modeling as illustrated in Figure 3 (Wang et al. SNMMI 2018). Standard compartmental modeling (Fig. 3A) in dynamic PET commonly assumes a time-invariant model (Figure 3B). This assumption can work well for dynamic PET of standard temporal-resolution but no longer meets the need for modeling of HTR data. The new time-varying model divides a tracer uptake period into a vascular phase and a tissue uptake phase to better model the fast perfusion kinetics (Fig. 3C). An advantage of this time-varying modeling is that blood flow (denoted as F) then can be estimated independently of radiotracer extraction fraction. This method has the potential to enable the classic metabolic radiotracer 18F-FDG (and potentially many other radiotracers) for quantitative perfusion imaging, thus allowing multiparametric imaging using a single tracer. Furthermore, the new modeling method can also separate glucose transport rate K1 from blood flow F (Wang et al. SNMMI 2019), while they are mixed with each other in the standard model. This provides a new opportunity to better quantify radiotracer transport kinetics. 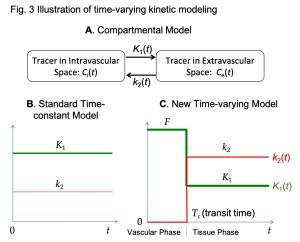
Publications
Wang GB.
High temporal-resolution dynamic PET image reconstruction using a new spatiotemporal kernel method.
IEEE Transactions on Medical Imaging, 38(3): 664 – 674, 2019.
[Fully3D 2017; bioRxiv 2018]
Wang GB, Sarkar S, Kim E, Badawi RD,
Time-varying kinetic modeling of high temporal-resolution dynamic 18F-FDG PET data for multiparametric imaging,
Journal of Nuclear Medicine (2018 SNMMI Annual Meeting), 59 (supplement 1): 503, 2018. (abstract)
Wang GB, Spencer B, Sarkar S, Shi H, Chen S, Hu P, Ding Y, Hu D, Zhou P, Xu T, Wang C, Jones T, Cherry SR, Badawi RD.
Quantification of Glucose Transport Using High Temporal Resolution Dynamic PET Imaging.
Journal of Nuclear Medicine (2019 SNMMI Annual Meeting), 60 (supplement 1): 521, 2019. (abstract)